

NCT03274219 : Phase 1 - bb21217 an Anti-BCMA CAR T Cell Drug Product, in RRMM - CRB-402
CRB-402 Study of bb21217 in Multiple Myeloma Relapsed Refractory Multiple Myeloma RRMM Study CRB-402 is a 2-part, non-randomized, open...
Dec 29, 2017


NCT03158688: Phase 3 - Carfilzomib, Daratumumab and Dexa for relapsed Multiple Myeloma. (CANDOR)
Phase 3 CANDOR study Dara-Kd Kd Study of Carfilzomib, Daratumumab and Dexamethasone for Patients With Relapsed and/or Refractory Multiple...
Dec 28, 2017


NCT03151811: Phase 3- Melphalan Flufenamide (Melflufen)-Dex or Pom-dex for RRMM ref. to Len. (OCEAN)
OCEAN NCT03151811: Phase 3: A Study of Melphalan Flufenamide (Melflufen)-Dex or Pomalidomide-dex for RRMM Patients Refractory to...
Dec 17, 2017

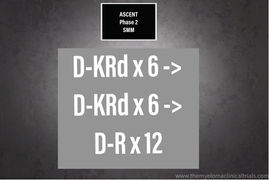
NCT03289299 : Phase 2 - Agg. Smoldering Curative Approach Eval. Novel Therapies & Transplant ASCENT
ASCENT TRIAL Dara-KRd Aggressive Smoldering Curative Approach Evaluating Novel Therapies and Transplant (ASCENT) This study evaluates the...
Dec 16, 2017


NCT03319667: Phase 3 - Isatuximab, Bortezomib, Lenalidomide and Dexamethasone NDMM (IMROZ)
IMROZ Study NCT03319667 Clinical Benefit of SAR650984, Bortezomib, Lenalidomide and Dexamethasone Combination in NDMM Patients Not...
Dec 16, 2017


NCT03091257: Phase 1 - Dabrafenib and/or Trametinib in Patients With Relapsed Refractory Myeloma
Multiple Myeloma Research Consortium NCT03091257: Phase 1 - Dabrafenib and/or Trametinib in Patients With Relapsed and/or Refractory...
Dec 15, 2017


NCT03236428: Phase 2: Phase II Study of the CD38 Antibody Daratumumab MGUS / Smoldering Myeloma SMM
NCT03236428: Phase 2: Phase II Study of the CD38 Antibody Daratumumab in Patients With High-Risk MGUS and Low-Risk Smoldering Multiple...
Dec 15, 2017


NCT03374085: Phase 1/2: CC-92480 Monotherapy and with Dex in Relapsed / Refractory Multiple Myeloma
NCT03374085: Phase 1/2: CC-92480 Monotherapy and with Dex in Relapsed / Refractory Multiple Myeloma A Safety, PK and Efficacy Study of...
Dec 15, 2017


NCT03275103: Phase 1 - Dose-Escalation Study of Cevostamab in Participants With RRMM
NCT03275103: Phase 1 - Dose-Escalation Study of Cevostamab in Participants With Relapsed or Refractory Multiple Myeloma (R/R MM) A Phase...
Dec 14, 2017


NCT03318861: Phase 1: Study to Evaluate the Safety and Efficacy of KITE-585 in RRMM Multiple Myeloma
NCT03318861: Phase 1: Study to Evaluate the Safety and Efficacy of KITE-585 in Participants With Relapsed/Refractory Multiple Myeloma...
Dec 13, 2017


NCT03338972: Phase 1 - Autologous Anti-BCMA-CAR-expressing CD4+/CD8+ T-lymphocytes FCARH143 in RRMM
Immunotherapy With BCMA CAR-T Cells in Treating Patients With BCMA Positive Relapsed or Refractory Multiple Myeloma NCT03338972: Phase 1...
Dec 13, 2017


NCT03288493: Phase 1/2 - P-BCMA-101 Tscm CAR-T Cells in the Treatment of Patients With Myeloma
(PRIME) NCT03288493: Phase 1/2 - P-BCMA-101 Tscm CAR-T Cells in the Treatment of Patients With Multiple Myeloma (MM) NCT03288493: Phase...
Dec 13, 2017


NCT03145181: Phase 1 - Teclistamab, Humanized BCMA*CD3 Bispecific Ab, in Relapsed Myeloma MajesTEC1
MajesTEC1 Dose Escalation Study of Teclistamab, a Humanized BCMA*CD3 Bispecific Antibody, in Participants With Relapsed or Refractory...
Dec 12, 2017


NCT03218683: Phase 1/2: Study of AZD5991 Alone or with Venetoclax in Relapsed Hem Malignancies
NCT03218683: Phase 1/2: Study of AZD5991 Alone or in Combination With Venetoclax in Relapsed or Refractory Haematologic Malignancies....
Dec 10, 2017


NCT03119363: Treating Cancer-Related Fatigue Through Systematic Light Exposure
Cancer related fatigue (CRF) is the most common cancer side effect and can severely interfere with activities of daily living long after...
Dec 9, 2017


NCT03269136: Phase 1 -PF-06863135 As Single Agent & with Immunomodulatory Agents In relapsed Myeloma
NCT03269136 MAGNETISMM-1 RRMM NCT03269136: Phase 1 -PF-06863135 As Single Agent & with Immunomodulatory Agents In relapsed Myeloma To...
Dec 9, 2017


NCT03078452: Evaluating Effectiveness of Powered Drill Bone Marrow Biopsy - Multiple Myeloma
NCT03078452: Evaluating Effectiveness of Powered Drill Bone Marrow Biopsy Evaluating Effectiveness of Powered Drill Bone Marrow Biopsy...
Dec 9, 2017


NCT03070327: Phase 1 - BCMA Targeted CAR T Cells With or Without Lenalidomide for Multiple Myeloma
A Phase I Trial of B-cell Maturation Antigen (BCMA) Targeted EGFRt/BCMA-41BBz Chimeric Antigen Receptor (CAR) Modified T Cells With or...
Dec 8, 2017


NCT03314181: Phase 2 - Venetoclax, Daratumumab and Dex (+/- Bortezomib) in RRMM Multiple Myeloma
A Study of Combination Therapy With Venetoclax, Daratumumab and Dexamethasone (With and Without Bortezomib) in Participants With Relapsed...
Dec 8, 2017

