

NCT05558319: Phase 3: (GEM21menos65) Extended VRD Plus vs Isa-VRD vs Isa-V-Iberdomide - NDMM - ASCT
(GEM21menos65) (Isatuximab-VRD + ASCT) - (VRD extended + ASCT plus ERI) - (Isatuximab-VID + ASCT). ERI=Early Rescue Intervention...
Dec 1, 2022


NCT05519085: Phase 3: CC-92480 + Bortezomib-dex (480Vd) Vs Pom-Bortzomib-dex (PVd) RRMM -SUCCESSOR-1
(SUCCESSOR-1) CC-92480 - Mezi - Mezigdomide - CELMoD NCT05519085: Phase 3: A Study to Evaluate CC-92480, Bortezomib and Dexamethasone...
Dec 1, 2022


NCT05372354: Phase 1/2: CC-92480 (BMS-986348) in Combination With Other Treatments in RRMM Myeloma
CELMoD - CC-92480 - Mezigdomide - MEZI NCT05372354: Phase 1/2: A Study to Evaluate Safety, Drug Levels and Effectiveness of CC-92480...
Dec 1, 2022


NCT05552976: Phase 3: CC-92480 + Carfilzomib-dex (480Kd) Vs Carfilzomib-dex (Kd) RRMM (SUCCESSOR-2)
(SUCCESSOR-2) CC-92480 - Mezi - Mezigdomide - CELMoD NCT05552976: Phase 3: A Study to Evaluate CC-92480 in Combination With Carfilzomib...
Dec 1, 2022


NCT04934475: Phase 3 - IFM 2020-02 Minimal Residual Disease Adapted Strategy (MIDAS) KRD-Isatuximab
IFM 2020-02 MInimal Residual Disease Adapted Strategy (MIDAS) Isa-KRd NCT04934475: Phase 3 - IFM 2020-02 -MInimal Residual Disease...
Dec 30, 2021


NCT05064787: Evaluating the Feasibility of a Digital Health Coaching Program Post CART
NCT05064787: Evaluating the Feasibility of a Digital Health Coaching Program for Individuals Following CAR T Therapy The aim of this...
Dec 15, 2021


NCT04855136 : Phase 1/2 - Safety and Efficacy of bb2121 (Ide-cel) Combinations in MM (KarMMa-7)
bb2121 Safety and Efficacy of bb2121 (Ide-cel) Combinations in Multiple Myeloma (KarMMa-7) KarMMa-7 Relapsed & Refractory Multiple...
Dec 1, 2021


NCT04975997: Phase 3: IberDd Versus DVd in Participants With Relapsed Refractory Myeloma - EXCALIBER
Open-label Study Comparing Iberdomide, Daratumumab and Dexamethasone (IberDd) Versus Daratumumab, Bortezomib, and Dexamethasone (DVd) in...
Aug 6, 2021


NCT04975399: Phase 1: Study to Evaluate the Safety and Tolerability of CC-92328 in Participants RRMM
NCT04975399: Phase 1: Study to Evaluate the Safety and Tolerability of CC-92328 in Participants With Relapsed and/or Refractory Multiple...
Aug 6, 2021


Expanded Access Protocol for Receiving Idecabtagene Vicleucel That is Nonconforming for commercial
NCT04771078: Expanded Access Protocol (EAP) for Participants Receiving Idecabtagene Vicleucel That is Nonconforming for Commercial...
Jun 12, 2021


NCT04776395: Phase 2: Iberdomide Alone or in Combination With Dex for Intermediate/High-Risk SMM
NCT04776395 Iberdomide Alone or in Combination With Dexamethasone for the Treatment of Intermediate- or High-Risk Smoldering Multiple...
Jun 10, 2021


NCT04674813: Phase 1 - A Study of CC-95266 in Subjects With Relapsed and/or Refractory Myeloma
A Study of CC-95266 in Subjects With Relapsed and/or Refractory Multiple Myeloma This is a Phase 1, multicenter, open label, study of...
Dec 27, 2020


NCT04392037: Phase 2: Iberdomide Combined With Low-dose Cyclophosphamide and Dexamethasone (ICON)
NCT04392037: Phase 2: Iberdomide Combined With Low-dose Cyclophosphamide and Dexamethasone (ICON) Iberdomide Combined With Low-dose...
Dec 18, 2020


NCT04635735: Phase 1/2: Study of Ipilimumab After Stem Cell Transplantation in People With RRMM
NCT04635735: Phase 1/2: Study of Ipilimumab After Stem Cell Transplantation in People With Relapsed/Refractory Multiple Myeloma...
Dec 18, 2020


NCT04555551:Phase 1 - MCARH109 Chimeric Antigen Receptor (CAR) Modified T Cells for Multiple Myeloma
MCARH109 CART MCARH109 Chimeric Antigen Receptor (CAR) Modified T Cells for the Treatment of Multiple Myeloma This study will test the...
Dec 17, 2020


NCT04287855: Phase 2: Phase 2 Study of Isatuximab + Pomalidomide, Dex, Carfilzomib RRMM -IFM2018-03
IsKPd - IFM2018-03 NCT04287855: Phase 2: Open Label Phase 2 Study of Isatuximab Plus Pomalidomide and Dexamethasone With Carfilzomib in...
Dec 10, 2020


NCT04564703: EMN26 Phase 2: Iberdomide (Cc220) Maintenance After Asct in Newly Diagnosed MM Patients
EMN26 NCT04564703: Phase 2: Iberdomide (Cc220) Maintenance After Asct in Newly Diagnosed MM Patients Iberdomide (Cc220) Maintenance After...
Dec 9, 2020


NCT04394650: Phase 1 - CC-98633, BCMA-targeted Chimeric Antigen Receptor (CAR) T Cells, in RRMM
CC-98633 CART NCT04394650: Phase 1 - A Study of CC-98633, BCMA-targeted Chimeric Antigen Receptor (CAR) T Cells, in Subjects With...
Dec 1, 2020

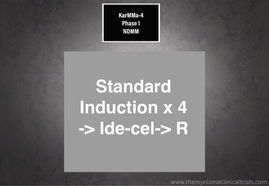
NCT04196491: Phase 1 - Evaluate the Safety of bb2121 in High Risk, New Myeloma (NDMM) (KarMMa-4)
KarMMa-4 A Study to Evaluate the Safety of bb2121 in Subjects With High Risk, Newly Diagnosed Multiple Myeloma (NDMM) (KarMMa-4) Relapsed...
Dec 27, 2019

